
Microneedling Services In Charlotte NC
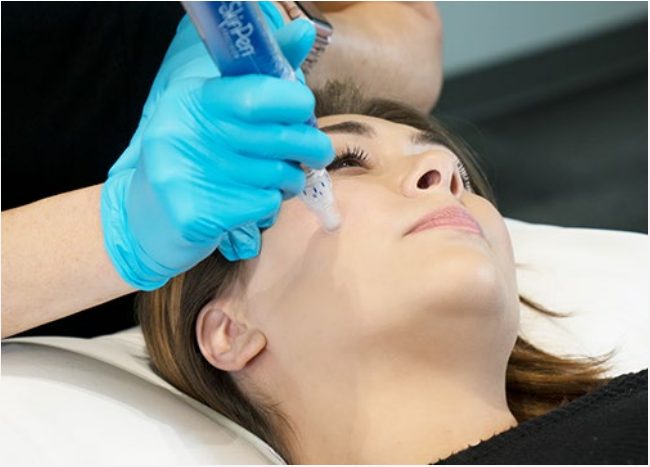
SkinPen by Bellus Medical is the first FDA-cleared microneedling device.
It causes controlled micro-injuries that stimulate your body’s natural wound healing process, while minimizing cellular damage. The result is effective remodeling of scar tissue, while keeping the overall structure of the skin intact.
There are three phases to the wound-repair process:
- Phase I: Inflammation. Piercing the skin triggers your immune system to disinfect the wounds, remove debris, increase blood flow and begin to create new tissue.
- Phase II: Proliferation. The wound is rebuilt with new granulation cells, which are part of the extracellular matrix. Additionally, a new network of blood vessels develops.
- Phase III: Remodeling. The wound is replaced with new dermal tissues and blood vessels. Best of all, SkinPen works. Ninety percent of subjects in the clinical trial would recommend the procedure to family and friends.
References
[1] Data on file. Bellus Medical. 2017.
Frequently Asked Questions
What About SkinPen Did the FDA ‘Clear’?
Not All Microneedling Is The Same – The First Class II FDACleared Microneedling Device. SkinPen Precision underwent a very rigorous, 90 point validation study conducted by a 3rd party which set a new, superior standard for microneedling devices. As a result, SkinPen was the 1st FDA-cleared device and the winner of multiple device design awards. The results speak for themselves.
What Is SkinPen®?
SkinPen by Bellus Medical is the first FDA-cleared microneedling device in the world, clinically proven to safely and effectively treat facial acne scars for ages 22 and up. With as few as three non-invasive and affordable treatments spaced 30 days apart, you can improve your appearance for six months after your last treatment – and step out with confidence.
Why Use It?
SkinPen is clinically proven to reduce the appearance of acne scars. In fact, 90% of subjects in the clinical trial would recommend the treatment to friends and family. It’s a minimally invasive procedure performed in-office with little downtime. As the first FDA-cleared microneedling device, SkinPen sets the industry standard for safety.
Will It Work for Me?
Unlike some alternatives, SkinPen has been clinically proven to be effective for women and men with skin ranging from light to very dark. 1 Likewise, unlike lasers or chemical peels that can damage skin over the long term, SkinPen treatments, when properly spaced and overseen by a physician, can be used for years.
How Does It work?
SkinPen creates hundreds to thousands of “micro” skin punctures per second to stimulate the skin’s natural wound healing process – inflammation, proliferation, and remodeling – to prompt tissue remodeling without causing scar tissue formation. 2 Most patients can return to normal activities within 24 hours.
How Can I Make It More Effective?
While SkinPen treatments are effective on their own, you might be able to see additional results with Bellus Medical’s Skinfuse® Post-Treatment Kit. The 90-day combination of PURIFY Cleansing Complex, RESCUE Calming Complex, FORTIFY Vita C Serum, RECLAIM Hydrating Support, and SHIELD Zinc Oxide SPF 30 optimizes the results of microneedling.

Is It Safe?
Yes, by design. SkinPen’s patent-pending – and single-use – sterile needle cartridge is built with safety in mind. SkinPen is also surrounded by a proprietary BioSheath that acts as a barrier to prevent cross contamination between procedures. 1 That’s part of the reason SkinPen by Bellus Medical is the world’s first FDA-cleared microneedling device. Learn more by reviewing our patient labeling document.
Who Should Not Use It?
SkinPen should not be used on patients who have active skin cancer in the treatment area(s); open wounds, sores, or irritated skin in the treatment area(s); an allergy to stainless steel or anesthetics; a hemorrhagic (bleeding) disorder or hemostatic (bleeding) dysfunction; are pregnant or nursing; or are currently taking drugs with the ingredient isotretinoin (such as Accutane).
References
[1] Data on file. Bellus Medical. 2017. [2] Data on file. Bellus Medical. 2016
